
The US FDA New Drug Approvals in January 2025
Shots:
-
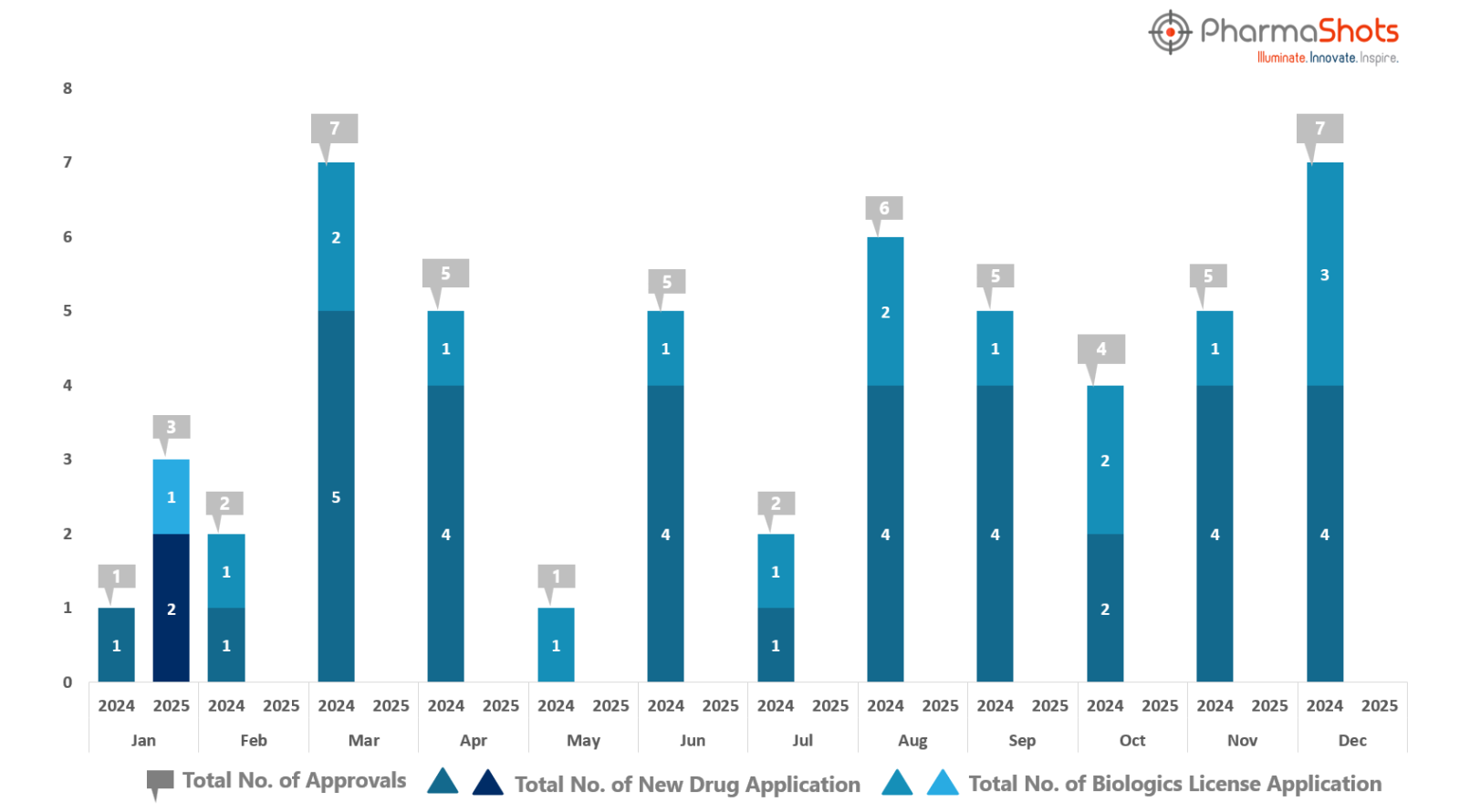
PharmaShots has compiled a list of US FDA-approved drugs in the month of January 2025
-
The US FDA has approved a total of 3 new drug including 2 new molecular entities and 1 biologic leading to the treatment of patients and advances in the healthcare industry
-
The major highlighted drug was Daiichi Sankyo & AstraZeneca’s Datroway securing approval for treating unresectable or metastatic HR+/HER2- Breast Cancer
Company: Daiichi Sankyo and AstraZeneca
Product: Datroway
Active Ingredient: Datopotamab Deruxtecan-dlnk
Disease: Unresectable or Metastatic HR+/HER2- Breast Cancer
Date: Jan 17, 2025
Shots:
-
The FDA has approved Datroway to treat unresectable or metastatic HR+/HER2- (IHC 0, IHC 1+ or IHC 2+/ISH-) breast cancer in patients receiving endocrine-based therapy & CT. Submissions are under review in the EU, China, & other areas
-
Approval was based on a global P-III (TROPION Breast01) trial, assessing Datroway (6mg/kg; IV, Q21D) vs single agent CT (eribulin, capecitabine, vinorelbine or gemcitabine) in HR+/HER2- breast cancer patients (n=732)
-
The trial showed improved PFS of 37%, mPFS (1EP; 6.9 vs 4.9mos.), ORR (2EPs; 36% vs 23%) with CR (2; 0.5% vs 0; 0%) & PR (131; 36% vs 84; 23%), plus mDoR (2EPs; 6.7 vs 5.7mos). Safety was favorable in 360 subjects & data was published in JCO
Company: Axsome Therapeutics
Product: Symbravo
Active Ingredient: Meloxicam and Rizatriptan
Disease: Migraine
Date: Jan 30, 2025
Shots:
-
The US FDA has approved Symbravo for acute treatment of migraine with/without aura in adults based on P-III (MOMENTUM), P-III (INTERCEPT) & P-III (MOVEMENT) trial; commercially available in ~4mons.
-
MOMENTUM trial assessed Symbravo (single dose) vs PBO/meloxicam/rizatriptan, showing greater pain & symptoms freedom (photophobia, phonophobia & nausea) at 2hrs., sustained through 24-48hrs., 77% pts don't require rescue therapy within 24hrs. of dosing compared to rizatriptan from 2-24hrs.
-
INTERCEPT trial also showed similar results, with 85% pts not requiring rescue therapy within 24hrs., while MOVEMENT trial showed its long-term safety in 706 pts, dosed intermittently for ~12mos. & treating ~2 migraines/mos.
3. Vertex Receives the US FDA Approval for Journavx (Suzetrigine) to Treat Acute Pain
Company: Vertex Pharmaceuticals
Product: Journavx
Active Ingredient: Suzetrigine
Disease: Moderate-to-Severe Acute Pain
Date: Jan 30, 2025
Shots:
-
The FDA has approved Journavx (50mg; BID) to treat moderate-to-severe acute pain in adults
-
JOURNAVX is an oral, non-opioid pain signal inhibitor that selectively targets NaV1.8 relative to other NaV channels
-
The company is also testing suzetrigine for peripheral neuropathic pain (PNP), with ongoing P-III trial for diabetic neuropathy and plans for lumbosacral radiculopathy studies, subject to regulatory discussions
Related Post: Insights+: The US FDA New Drug Approvals in December 2024
Tags

A passionate content writer with expertise in delivering high-quality and engaging content, Dipanshu is a keen reader and a versatile writer. Dipanshu dedicatedly covers news ranging from biopharma, life sciences, biotech, and MedTech to diagnostics and animal health companies, FDA, EMA, and biosimilar approvals. He can be contacted at connect@pharmashots.com














